Esophageal Carcinoma:
Esophageal cancer is the 3rd most common GI malignancy [31,35].
Esophageal cancer is responsible for about 13,000 deaths annually
in the United States [15]. More than 90% of esophageal cancers are
either squamous cell or adenocarcinomas [39]. The prevalence of
esophgeal cancer has increased dramatically over the past 30 years
(350-800% increase in adenocarcinoma) [1,7,30]. Squamous cell
carcinomas are the most common esophageal neoplasm worldwide and
still account for about 70-85% of cases, adenocarcinoma now
surpassess SCC as the predominant histologic type in the United
States [1,7,30,39]. Squamous cell carcinoma is more common in men
(about 65% of patients), usually arises in the mid or upper
one-third of the esophagus, and the prevalence increases with age
(peak 60-74 years) [39,41]. In the US, black males are at the
highest risk with a rate 4 times that of white men [39]. Patients
typically present with dysphagia, odynophagia, and weight loss
[39]. For adenocarcinoma, approximately 85% of patients are men
and the peak incidence is in the 7th decade [39]. In contrast to
SCC, esophageal adenocarcinoma is about 5 times more common in
white men than blacks [39]. Esophageal adenocarciomas are most
commonly found in the distal esophagus and have a marked tendency
to invade the gastric cardia and fundus by direct extension
[39,41].
The greatest risk factors for the development of esophageal carcinoma are ETOH abuse and smoking [22,39]. Other risk factors include achalasia (carcinoma develops in 5-10% of patients due to stasis- risk is 30 fold greater than the general population), acid or lye strictures, Barrett's esophagus (adenocarcinoma is seen in 10%), hereditary tylosis (familial hyperkeratosis- a hereditary disorder secondary to a defect in the tylosis esophageal cancer gene on chromosome 17q25), Plummer-Vinson syndrome (characterized by dysphagia, iron deficiency anemia, and upper esophageal webs), celiac disease, and previous head and neck tumors (10% of patients with esophageal carcinoma have a head and neck squamous cell carcinoma) [39].
Squamous cell cancers are evenly distributed between the middle
and lower esophagus (less commonly the upper third), whereas about
3/4's of adenocarcinomas are found in the distal esophagus
[31,39]. Early stage esophageal cancer is often asymptomatic and
detection is uncommon [3]. As a consequence, esophageal cancer
usually presents at an advanced stage with 75% of patients having
lymph node metastases at presentation [30]. Overall survival rate
at 5-years is 10%-25%, however, patients with early stage cancer
can have survival rates between 57% to 78% [3,6,7]. By histologic
staging the 5 year survival following advanced treatment
modalities is 78% for stage 0, 68% for stage I, 60% for stage II,
34% for stage III, and 16% for stage IV [29].
Esophageal adenocarcinoma has an overall better long-term
prognosis after resection than does squamous cell cancer (5-year
survival of 47% versus 37%, respectively) [31]. Independent of
staging, esophageal adenocarcinoma has a better prognosis than SCC
with a 5-year survival rate after resection of 42% [39]. Note-
that patients with adenocarcinoma of the esophagogastric junction
and gastric cardia have lower survival rates than patients with
other forms of gastric cancer [41]. Histologic grade (G1-G3) also
affects survival with increased histologic grade associated with
an incrementally decreased survival [41].
The two most important prognostic indicators for esophageal carcinoma are depth of tumor penetration and nodal involvement [7,25,39]. Esophageal carcinoma is staged based upon a TNM classification system [7]. A higher T stage is associated with a greater likelihood of nodal and metastatic disease [41]. Invasion limited to the mucosa (T1) is associated with a 5-year survival rate of more than 80% [39]. For patients with esophageal tumors remaining in the wall of the esophagus the 5-year survival is approximately 40% [7]. If the tumor invades the adventitia (T3), survival drops to 4% at 5 years [7]. Patients with advanced, but potentially resectable tumors (T1/N1-3/M0 and T2-4a/N0-3/M0) generally receive neoadjuvant chemotherapy, radiation therapy, or both followed by surgery which results in improved disease free survival (5 year survival is 20-30%; other authors suggest adjuvant therapy improves 5 year survival from 34% to 47% [45]) [6,8,45,47]. Patients with lymph node metastases have a 5 year survival of only 3%, compared to 40-42% without nodal involvement [1,7,30]. Distant metastases are found in about 18% of patients at presentation- especially to abdominal nodes, liver, and lung. Hematogenous metastases most commonly involve the liver, lungs, and bones, followed by the adrenal glands, kidneys, and brain [25].
Treatment for esophageal carcinoma depends on accurate staging [6]. In general, stages I-III are treated with surgery, as well as chemotherapy and radiation [30]. Stage IV (distant metastases) is inoperable and chemotherapy and radiation are used for palliation [30]. For curative surgical resection, the upper resection margin should be 8-10 cm above the tumor, and the lower margin more than 5 cm below the tumor [31]. Surgery for operative candidates is not without significant risk and is associated with a mortality between 5 to 20% [1]. Moreover, the overall survival after "curative" resection does not exceed 25% which indicates micrometastases or undetected metastases were likely present at the time of surgery [10]. Proper identification of patients that are truly surgical candidates is crucial so that patients with advanced stage disease can be referred for prompt multimodality treatment protocols [3]. Neoadjuvant chemotherapy or chemoradiation prior to surgical resection (trimodal therapy) is the mainstay for management of patients with advanced disease [21,44,46]. Organ preserving selective resection following definitive chemoradiation therapy has been shown to have a promising efficacy with a reported 7-year overall survival rate of 31.7% [46]. Pathologic complete response following neoadjuvant therapy is achieved in 25-42% of patients and is associated with a lower rate of recurrence and longer survival [47]. However, up to 60% of tumors show either minimal or no pathologic response following neoadjuvant chemotheapy and 30-40% for chemoradiation therapy [44]. Additionally, some studies have suggested no overall survival benefit with the addition of surgery to patients that demonstrated a clinical response following chemoradiotherapy (although locoregional recurrence was higher in these patients, however, treatment related mortality was higher in patients that also received surgery) [46]. Because the heart may be included within the radiaiton port, complication can include acute and chronic pericarditis, coronary artery disease, conduction abnormalities, and cardiomyopathy [21].
Although endoscopic evaluation and CT imaging form the mainstays of clinical staging, they cannot accurately identify all sites of disease [3]. FDG PET imaging holds great promise as a means to fully assess for the presence of unsuspected sites of disease in patients presenting with esophageal carcinoma. PET in particularly useful in reducing the number of unnecessary surgical procedures through the identification of unsuspected sites of disease [10]. Up to 20% of patients can be upstaged due to the presence of metastatic disease identified on PET imaging [10]. FDG PET has been shown to be superior to FLT PET for the evaluation of esophageal cancer [12].
FDG PET imaging is presently reimbursed for the following indications:
1- Pre-surgical staging and restaging of esophageal carcinoma
2- Monitoring for recurrence (may be covered unless strong negative evidence is present)
Primary tumor (T-stage):
Primary resection is possible when the primary tumor is confined
to the esophageal wall (T1-T2) [28]. Extension into the
periesophageal adventitia (T3) is potentially resectable when
treated with combined modality therapy [28]. Invasion into
adjacent organs (aorta, diaphragmatic crus, or liver) indicates T4
disease [28]. A higher T classification is associated with a
greater likelihood of nodal metastatic disease and poorer
long-term prognosis [28,41]. CT is unable to adequately
differentiate between T1, T2, and T3 disease [31]. Loss of the fat
plane between the esophagus and adjacent structures or
displacement or indentation is suggestive of T4 disease [31].
However, endoscopic US is more accurate than CT and the modality
of choice for assessing the depth of penetration of the primary
tumor through the esophageal wall at initial staging [28,41]. On
endoscopic US, the esophageal wall is visualized as 5 alternating
layers of differing echogenicity [31]. The average accuracy of
endoscopic US for T-staging is 84% [31].
For CT, pericardial invasion is suspected if there is
obliteration of the fat plane between the esophageal mass and
pericardium, pericardial thickening, pericardial effusion, or
indentation of the heart with a concave deformity [41].
Tracheobronchial invasion should be suspected if there is a
discrete indentation on the posterior wall or displacement of the
trachea or bronchus by the tumor [41]. Aortic invasion is
suggested by greater than 90 degrees of contact between the tumor
and the aorta or if there is oblietration of the traingular fat
space between the esophagus, aorta, and spine [41].
PET/CT has been shown to be of incremental value in the primary staging of esophageal cancer [36]. Despite reported sensitivities between 91-100% for demonstrating primary esophageal cancer (both squamous cell and adenocarcinoma), FDG PET imaging lacks sufficient spatial resolution and boundary determination to accurately assess T-stage [9,10,23]. False negative exams occur in up to 20% of cases including of carcinoma-in-situ, T1 (small) cancers, and well-differentiated adenocarcinomas [6,10,24]. False positive exams can occur due to esophageal inflammation and there can be normal mild FDG activity in the esophagus possibly due to swallowed saliva or smooth muscle metabolism which could potentially obscure subtle lesions [1]. PET imaging is also not sensitivity for assessing for the presence of local invasion [1].
Findings on the PET/CT exam can change patient stage in up to 40%
of patients and change management in up to 34% of cases [36]. PET
exam results can also provide prognostic information [14].
Patients with high SUV values have a worse survival rate, however,
this is not an independent predictor for survival which is also
strongly affected by eligibility for surgery [14,36]. In one
study, the five year survival for patients staged by PET/CT were
40% (stages I-IIA), 38% (stage IIB-III), and 6% for stage IV [36].
Evidence is emerging that tumor textural analysis can yield
additional prognostic information superior to SUVmax (which is
derived from a single voxel and does not characterize the entire
tumor biology) [43,45]. These textural features are felt to
reflect underlying biologic features of the tumor such as
vascularization, cell density, tumor aggressiveness, or hypoxia,
which in turn may be related to the degree of chemoradiation
resistance [43]. In one study, tumors which demonstrated
heterogeneity on textural analysis were less likely to reach a
complete pathologic response compared to homogeneous tumors [43].
Nodal metastases:
Staging of nodal metastases in esophageal cancer is either N0 (absent) or N1, N2, N3 (positive for locoregional nodal metastases) [28]. Locoregional nodes are those that represent sites of primary lymphatic drainage and are normally resected with the primary tumor at the time of esophagectomy [28]. The definition of which nodes constitute locoregional nodes depends on the location of the primary tumor (upper, middle, lower esophagus) [28]. The lymphatic drainage of the upper two-thirds of the esophagus tends to be upward and that of the distal esophagus tends to be downward- although bidirectional drainage is common [31]. For cervical esophageal tumors locoregional nodes include scalene, internal jugular, and supraclavicular nodes [28]. For tumors in the mid-thoracic esophagus locoregional nodes include periesophageal and subcarinal nodes (metastases to cervical or celiac axis nodes would be considered M1b disease in these patients) [28,31]. For tumors in the lower thoracic esophagus the lymphatic drainage follows the blood supply of the left gastric artery to the left gastric lymph nodes and lymph nodes in the gastrohepatic ligament and these would be considered resectable (N1) [28]. Previously, for lower thoracic tumors, metastases to celiac nodes were considered M1a disease (non-resectable) [28], but this is now considered nodal metastatic disease [41]. For tumors arising from the GE junction region local nodes include lower periesophageal nodes, pulmonary ligament nodes, diaphragmatic nodes (lying on the dome of the diaphragm or in the retrocrural region), pericardial lymph nodes (located immediately adjacent to the gastroesophageal junction), left gastric lymph nodes, and celiac lymph nodes [28]. Nodal metastases outside of these designated areas were previously considered M1a disease- "non-regional nodal mets" [28]. Patients with M1a disease had a much worse prognosis compared to patients with only locoregional nodal involvement, but have a better prognosis than patients with distant organ mets (M1b) [28]. Non-regional (distant) lymph node metastases without involvement of intervening locoregional nodes can occur in up to 25% of cases [28].
Metastases to lymph nodes is one of the most important prognostic
factors in patients with esophageal cancer [6]. For patients with
lymph node metastases at presentation, the 5-year survival is
3-18%, whereas patients without nodal involvement have a 5-year
survival of 37-42% [30,36]. Both the number and the location of
involved lymph nodes affect prognosis [6]. Patients who have less
than 3 to 5 regional nodal metastases survive appreciably longer
than those who have more than 10 involved nodes [15]. It appears
that PET detected metastatic nodal disease carries a worse
prognosis compared to patients with nodal disease that is
undetectable on PET [36].
FDG PET is more sensitive than CT for depicting nodal metastases in patients with esophageal cancer [6]. CT has a sensitivity of 31%-44% for detecting N1 nodes and 14%-29% for detecting cervical or upper abdominal nodes, compared to 55%-64% and 43%-71%, respectively, for FDG PET imaging [6,10]. CT has an accuracy of 40-73% for the detection of pathologic mediastinal nodes (using a 1 cm size criteria) [1,6]. The reported accuracy of FDG PET in the staging of locoregional lymph node metastases varies from 24-90% [1,6]. Some of this variability in the accuracy of PET imaging is related to the limited spatial resolution of PET imaging and local nodal metastases adjacent to the primary tumor may be obscured by tumor activity [1,9,10]. Additionally, para-esophageal nodes are usually small in size and may contain only microscopic foci of tumor [9]. These nodes are generally best depicted by trans-esophageal ultrasound [10]. PET/CT has also been shown to improve detection of locoregional nodes by providing a high-resolution image of crowded para-esophageal structures- increasing the sensitivity to almost 94% compared to 82% for PET alone [20]. Overall, the greater the SUV in the primary tumor (SUV >5), the more likely lymph node metastases will be detected [38]. PET/CT also improves the negative predictive value by better characterization of sites of physiologic tracer activity [20].
Endoscopic US is superior to CT and PET/CT in the detection of
locoregional node metastases in patients with esophageal cancer
[28,41]. The accuracy of US for preoperative N-staging
ranges from 72-92% [31,39]. However, although the presence of
local para-esophageal nodal metastases carries prognostic
information, the presence of these metastases does not preclude
curative surgical resection (for example, they do not
necessarily change clinical management) [1]. However, supraclavicular, cervical, and celiac
lymph node metastases were previously considered M1a disease
[1,41]. The presence of pathologic adenopathy in these other
sites would substantially alter patient management and these
sites of disease are more accurately detected with PET imaging
[6,10].
Despite comprehensive assessment, between 11-56%
of clinically N0 patients with esophageal cancer are found to
have region lymph node metastases after esophagectomy [40].
Findings that suggest an increased risk for occult LN metastases
include an SUV max =/> 4.8 and higher clinical stage (cT2-4)
[40]. Importantly, in up to 46% of patients, occult LN
metastases are not always located in the vicinity of the primary
tumor suggesting that lymph node dissection should be performed
carefully and with sufficient range- particularly in patients at
high risk for occult LN metastases [40].
|
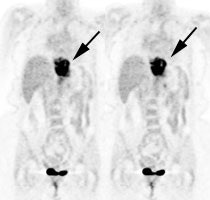
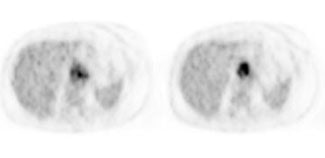
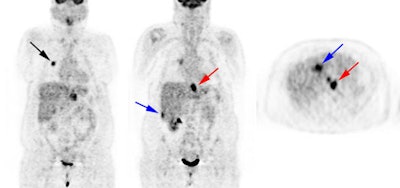
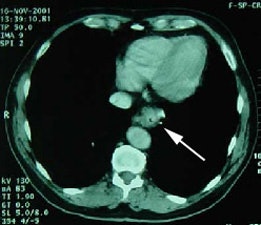
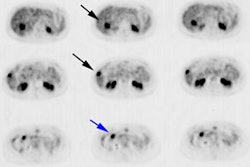
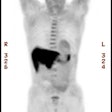
Esophageal carcinoma: The patient shown below presented with a history of progressive dysphagia. A barium swallow revealed a distal esophageal mass. CT and FDG PET imaging were performed for patient staging. The large esophageal mass (white arrows on CT) demonstrated intense tracer uptake (black arrows on PET scan). Uptake could also be seen in regional gastrohepatic ligament nodal metastases (right images). Retrocrural metastases seen on CT blended imperceptibly with the primary tumor on PET imaging. The was no evidence of distant metastatic disease. (Click here to view rotating volume image [1.5 MB])
|
|
|
Distant Metastases:
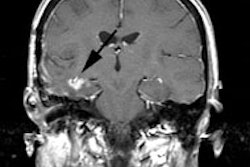
Approximately 20-30% of patients with esophageal carcinoma have distant metastatic disease at the time of presentation [15,28]. The most common visceral metastatic sites include (in decreasing prevalence) the liver (35%), lung (20%), bone (9%), adrenal glands (5%), kidneys, and brain [15,31,41]. Brain metastases are found in less than 2% of patients with metastatic disease [15]. The likelihood for the presence of distant metastases increases with the T-stage of the tumor- particularly with T3 lesions [10]. The presence of distant organ metastases (M1b disease) in esophageal cancer has considerable impact on patient management, as patients are then considered non-resectable. Unfortunately, over 30% of distant metastases are radiographically occult [10]. The major advantage of FDG PET over conventional imaging is the ability to detect distant metastases [1,10]. In a prospective study of 100 patients with esophageal carcinoma referred for surgery, FDG PET imaging was shown to be more accurate than CT in detecting distant metastases (84% versus 63%) [4].
FDG PET imaging has been shown to have higher accuracy for staging of M1a lymph nodes and is also more accurate than CT in presurgical staging for the presence of resectable versus nonresectable disease [27]. FDG-PET has a reported sensitivity of 69%-100%, a specificity of 84%-90%, and an accuracy of 84%-91% for the evaluation of distant metastases [1, 3, 4,10]. Sensitivity of CT for distant metastases has been reported to be as low as 30%-46% [4,10].
FDG-PET can demonstrate metastatic disease not identified on conventional studies in about 9% to 28% of patients [1,3,4,5,15,31]. In the ACSOG trial, PET detected unsuspected biopsy proven metastatic disease in 4.8% of patients following a negative conventional workup [23]. However, in that same study, an additional 9.5% of patients had apparent metastatic disease on the PET exam that the surgeon felt did not require confirmation - hence, the overall rate of unsuspected metastatic disease may have been as high as 14.3% [23]. However, the diagnostic yield of PET in the detection of unsuspected metastatic disease in early stage (Tis or T1) cancer is very low and probably not indicated in these patients [31].
PET exams can also exclude metastatic disease at sites considered abnormal on conventional imaging. FDG PET exam results can affect patient management in approximately 22% of patients [5]. Metastatic lesions not identified on FDG-PET exams are typically less than 1 cm in size and are usually in the lung or liver [4]. ABout 5% of patients will have metastatic disease that will not be detected on either conventional or PET imaging [23].
PET imaging also provides prognostic information. When PET exams demonstrate only local disease, the 30-month survival has been reported to be up to 60%; while patients with distant disease on PET exams had a 30-month survival of 20% [4]. Other findings on the PET exam that are associated with a worse prognosis include increasing maximum SUV of the primary tumor, tumor length on the PET scan (over 5 cm), number of PET-positive nodes, and increasing PET stage [11,19].
|
Metastatic esophageal carcinoma: The patient presented for evaluation of a pulmonary nodule. PET revealed tracer uptake in the nodule (black arrow), as well as in an esophageal mass (red arrow), para-esophageal adenopathy, and liver metastases (blue arrows). |
|
|
|
Unsuspected metastatic esophageal carcinoma: The patient shown in the case below was referred for FDG PET imaging for the evaluation of esophageal carcinoma. A CT scan demonstrated abnormal thickening of the distal esophagus (white arrow), but no evidence of metastatic disease. The PET exam revealed long segment abnormal increased tracer activity within the primary esophageal lesion, as well as an unsuspected bone metastasis to the right hip (black arrow). Such findings on PET imaging have tremendous impact on patient management. Case courtesy of CTI.
|
|
|
Synchonous tumors:
Synchronous tumors (often of the head and neck) are not uncommon in patients with esophageal carcinoma [13]. PET imaging can detect unsuspected synchronous primary neoplasms in 1.5% to 5.5% of patients during initial staging for esophageal carcinoma [13,28].
Response to therapy:
Neoadjuvant chemotherapy alone or in combination with radiation
therapy is used for the treatment of locally advanced esophageal
carcinoma [33]. Neoadjuvant therapy is aimed at shrinkage of the
primary tumor and the eradication of metastases to improve
survival and the likelihood of surgical resectability [16,34]. In
patients with locally advanced disease without distant metastases
esophagectomy is a potential treatment option after successful
neoadjuvant chemo-radiation therapy and the use of pre-operative
chemoradiation has been shown to increase overall survival
compared to surgery along [28,34] (neoadjuvant therapy has been
shown to be associated with a 7-13% survival benefit compared to
surgery alone [42]).
Despite neoadjuvant therapy, the overall 5-year survival rate of
patients with esophageal cancer remains relatively poor (34-47%)
[42]. The low survival rates are mainly attributable to the high
incidence of recurrent disease early after treatment (ranging from
45-53%) [42]. Most recurrences occur within the first 2 years
after surgery with a median time to recurrence of 10-12 months
[42]. After recurrence is diagnosed, the median survival rate is
poor (3-9 months) [42].
The maximum improvement in terms of increased survival from
neoadjuvant treatment is observed for patients who achieve a
complete pathologic response [35,43]. Unfortunately, not all
patients will respond to chemotherapy [34]. Up to 60% of tumors
show either minimal or no pathologic response following
neoadjuvant chemotheapy and 30-40% for chemoradiation therapy
[44]. A complete histopathologic response- defined as less than
10% residual viable tumor cells, is achieved in 25-50% of patients
and complete pathologic response after induction therapy is
associated with a cure rate of approximately 60% [33,43] (15-30%
have no residual cancer cells in the primary tumor or lymph nodes
[35]).
FDG PET imaging can be used to evaluate response to neoadjuvant
therapy as CT imaging is generally ineffective for determining
pathologic tumor response (CT sensitivity 27-55% and specificity
50-91%) [5,15,16,18,31]. CT is limited because of difficulty in
distinguishing viable tumor from reactive inflammation or scar
tissue [31]. The PET response evaluation criteria in solid tumors
(PERCIST) appears to be superior to the RECIST criteria for the
evaluation of therapeutic response and prognosis in patients with
esophageal cancer receiving neoadjuvant therapy [37]. FDG PET
currently seems to be the best imaging modality for the assessment
of response to neoadjuvant therapy in esophageal cancer [31].
Early identification of patients that are not responding to
treatment would permit a change in the treatment regimen in order
to maximize the patients chances for cure and to minimize the side
effects of unnecessary chemotherapy [34].
A decrease in FDG uptake has been found to be significantly
greater in patients that are responding to therapy [5] and changes
in FDG uptake occur prior to any change in tumor size [18].
Effective assessment of tumor can be predicted as early as 14 days
following initiation of chemotherapy [18]. Various cutoffs
expressed as change in SUVmax have been used (35-78% [28,34,44]),
but generally a change of greater than 20-30% (PRECIST recommends
30% reduction in SUVmax) is considered to reflect a true
biological response [16,30,44]. The sensitivity and specificity of
serial FDG PET studies for determining therapy response are 71-85%
and 82-86%, respectively [8,16]. A recent meta-analysis found
a wide variability in reported sensitivities and specificities,
with a pooled sensitivity of 67% (95% CI 62-72%) and specificity
of 68% (CI 64-73%) [32]. Some of the variability in sensitivity
may be related to inclusion of different types of esophageal
cancer in many studies (squamous versus adeno), as well as
differences in neoadjuvant therapy regimens [32]. Nonetheless,
significantly longer overall and progression free survival has
been demonstrated in metabolically responding patients (using
various methods to define response such as an SUVmax <3 or a
decrease in SUVmax of 35%) [16,17,30,34,46]. With regards to other
quantitative data, a decrease in total lesion glycolysis of less
than 26% has shown to be associated with a poor pathologic
response (specificity 84% and PPV of 72% [49].
In patients receiving neoadjuvant therapy, interval metastatic
disease has been reported in 8-17% of cases [28]. The presence of
interval metastatic disease results in non-operable disease [28].
PET imaging permits detection of interval metastases that are
located outside the range of conventional imaging [28].
Primary tumor and nodal metastatic disease may demonstrate a
discordant response to neoadjuvant therapy [44]. Nodal metastases
may contain an aggressive subpopulation of cancer clones
originating from the primary tumor, which then evolve differently
at a genetic and phenotypic level [44]. It is these tumor
subclones that are likely responsible for local and distant
disease relapse [44].
Radiation therapy effects: Early PET imaging following radiation treatment or chemoradiation can result in a lower predictive accuracy in the proper identification of responders from non-responders [33]. This is likely the result of inflammatory changes associated with radiation therapy [33]. Radiation esophagitis is dose dependent [28]. In patients that are receiving radiation therapy, a 2 to 3 month delay is recommended prior to PET imaging to avoid false-positive exams associated with radiation esophagitis [5]. Because radiation esophagitis does not begin until after the first 2 weeks of treatment, evaluating treatment response prior to this time may be less prone to false-positive findings [28], although other authors still noted decreased accuracy even when scans were performed within 10 days of initiation of chemoradiation [33]. Radiation may induce FDG positive inflammation in the adjacent liver for patients with tumors in the distal esophagus and GE junction and this may be confused with metastatic disease [28].
Surgery performed within 4 weeks of the FDG exam can result in false-positive findings- it is recommended that scans be delayed for at least 6 weeks following surgery [8]. A mucosal biopsy can produce localized FDG uptake at the biopsy site [28].
PET/CT SUV measurements can be affected by respiratory motion
which are most significant at the level of the diaphragm
(calculated SUV max measurements can vary 30-50%) [28].
Incorporation of PET/CT techniques that correct for respiratory
motion artifacts are essential when acquiring images on patients
with distal or GE junction tumors [28].
A qualitative assessment scoring system has also been developed
[48]:
Score 1: No detectable focal uptake (complete metabolic response)
Score 2: Focal uptake greater than surrounding tissue or mediastinal blood pool, but less than liver (likely CMR)
Score 3: Diffuse uptake greater than MBP or liver (likely post treatment inflammation)
Score 4: Focal uptake greater than liver (likely residual tumor)
|
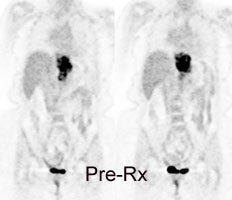
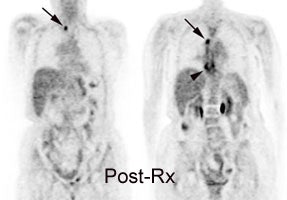
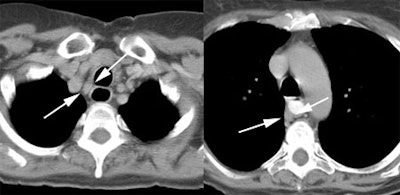
Response to therapy: This is the patient shown in the case above with progressive dysphagia and a large distal esophageal mass with gastrohepatic ligament adenopathy. Note the decreased uptake within the primary mass (arrowhead) and gastrohepatic nodes following initiation of radiation therapy. However, new uptake can be seen in two lymph nodes (black arrows) which were not identified prospectively on post-therapy CT imaging (white arrows on CT). The findings are concerning for metastatic disease and the patient went on to receive additional chemotherapy with subsequent resolution of the nodal uptake. |
|
|
Recurrent Esophageal Cancer:
Even after apparently curative surgery, the overall 5 year survival for esophageal carcinoma is only 15-50% [2,26]. Two-thirds of patients have a recurrence within one year and nearly all within 2 years after primary operation [2]. In about one-third to half of patients, the recurrence is located in the operative field (to regional lymph nodes or a recurrent mass) [2,6]. The remainder, and majority of recurrences are distant metastases [2]. Some reports suggest that early detection and aggressive treatment of recurrent disease may result in prolonged tumor-free survival and occasionally cure [2,26]. A multimodality approach is currently used for the detection of recurrent esophageal carcinoma [9]. FDG PET imaging allows a highly sensitive means for whole-body staging in patients with suspected recurrent esophageal carcinoma [2]. PET imaging can provide additional information in 27% of patients being evaluated for recurrent esophageal carcinoma [2]. Up to 12% of patients with negative conventional imaging exams can be shown to have recurrent disease by FDG-PET [2]. Additionally, the higher the SUV associated with recurrent disease, the worse the prognosis [26].
For the diagnosis of perianastomotic recurrence FDG-PET compares well with conventional imaging. Conventional imaging studies cannot reliably differentiate post surgical change/scar from tumor recurrence and FDG-PET is extremely useful in this setting [1]. In one study comparing PET and conventional imaging for the detection of recurrence, PET imaging had a sensitivity of 100%, specificity of 57%, and an accuracy of 74% (compared to 100%, 93%, and 96% for conventional imaging) [2]. By demonstrating increased metabolic activity within an abnormality detected by conventional imaging, a greater confidence in the diagnosis of recurrent disease can be achieved. Additionally, the findings on PET imaging can help to guide diagnostic biopsy to the regions of greatest metabolic activity.
For the diagnosis of regional and distant recurrences FDG-PET had a sensitivity of 94%, a specificity of 82%, and an accuracy of 87% (compared to 81%, 82%, and 81% for conventional imaging) [2]. For the evaluation of metastatic disease, FDG-PET had a sensitivity of 95%, compared to 79% for conventional imaging [2]. In another study, for the detection of recurrence at all sites, PET had a sensitivity of 93%, specificity of 76%, and an accuracy of 87% [26]. PET was particularly good for the detection of regional and distant recurrence [26]. At local sites, the sensitivity was high, but the specificity was lower (50%) due to a high incidence of false-positive findings [26].False-positive exams can be seen in association
with tracer uptake at the esophagogastric anastomosis [26] and
in association with recent endoscopic stricture dilatation (up
to 2 months post procedure) [2]. Because of the considerable
false-positive rate, histopathologic confirmation of PET
abnormalities suspicious for local recurrence should be performed [42].
|
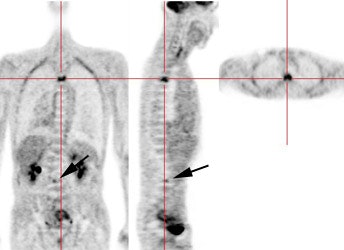
Recurrent esophageal cancer: The patient below had a history of esophageal cancer that had been treated with esophageal resection and gastric pull through procedure. The patient had a new pleural effusion and PET scan was performed as part of the evaluation for recurrent esophageal carcinoma. The PET scan revealed unsuspected bone metastases to the thoracic (cross-hairs) and lumbar spine (black arrow). |
|
|
REFERENCES:
(1) Radiographics 2000; Skehan S, et al. Imaging features of primary and recurrent esophageal cancer at FDG PET. 20: 713-723
(2) J Thorac Cardiovasc Surg 2000; Flamen P, et al. The utility of positron emission tomography for the diagnosis and staging of recurrent esophageal cancer. 120: 1085-92
(3) Thorax 1998; Lowe VJ, Naunheim KS. Current role of positron emission tomography in thoracic oncology. 53: 703-712
(4) Ann Thorac Surg 1999; Luketich JD, et al. Evaluation of distant metastases in esophgeal cancer: 100 consecutive positron emission tomography scans. 68: 1133-37
(5) Radiographics 2003; Kostakoglu L, et al. Clinical role of FDG PET in evaluation of cancer patients. 23: 315-340
(6) Radiology 2003; Yoon YC, et al. Metastasis to regional lymph nodes in patients with esophageal squamous cell carcinoma: CT versus FDG PET for presurgical detection- prospective study. 227: 764-770
(7) AJR 2003; Iyer RB, et al. Diagnosis, staging, and follow-up of esophageal cancer. 181: 785-793 (No abstract available)
(8) J Nucl Med 2004; Kostakoglu L, Goldsmith SJ. PET in the assessment of therapy response in patients with carcinoma of the head and neck and of the esophagus. 45: 56-68
(9) Radiology 2004; Rohren EM, et al. Clinical applications of PET in oncology. 231: 305-332
(10) J Nucl Med 2004; Heeren PAM, et al. Detection of distant metastases in esophageal cancer with 18F-FDG PET. 45: 980-987
(11) J Nucl Med 2004; Choi JY, et al. 18F-FDG PET in patients with esophageal squamous cell carcinoma undergoing curative surgery: prognostic implications. 45: 1843-1850
(12) J Nucl Med 2005; van Westreenen HL, et al. Comparison of 18F-FLT PET and 18F-FDG PET in esophageal cancer. 46: 400-406
(13) J Nucl Med 2005; van Westreenen HL, et al. Synchronous primary neoplasms detected on 18F-FDG PET in staging of patients with esophageal cancer. 46: 1321-1325
(14) AJR 2005; van Westreenen HL, et al. Prognostic value of the standardized uptake value in esophageal cancer. 185: 436-440
(15) Radiol Clin N Am 2005; Korst RJ, Altorki NK. Imaging esophageal tumors. 43: 611-619
(16) Radiology 2005; Westerterp M, et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy- systematic review. 236: 841-851
(17) Radiol Clin N Am 2005; Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. 43: 189-204
(18) J Nucl Med 2006; Wieder HA, et al. Comparison of changes in tumor metabolic activity and tumor size during chemotherapy of adenocarcinomas of the esophagogastric junction. 46: 2029-2034
(19) Ann Thorac Surg 2006; Rizk N, et al. Preoperative 18[F]-fluorodeoxyglucose positron emission tomography standardized uptake values predict survival after esophageal adenocarcinoma resection. 81: 1076-81
(20) J Nucl Med 2006; Yuan S, et al. Additional value of PET/CT over PET in assessment of locoregional lymph nodes in thoracic esophageal squamous cell cancer. 47: 1255-1259
(21) J Nucl Med 2006; Gayed IW, et al. The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. 47: 1756-1762
(22) AJR 2003; Iyer RB, et al. Diagnosis, staging, and follow-up of esophageal cancer. 181: 785-793
(23) J Thorac Cardiovasc Surg 2007; Meyers BF, et al. The utility of positron emission tomography in staging of potentially operable carcinoma of the thoracic esophagus: results of the American College of Surgeons Oncology Group Z0060 trial. 133: 738-745
(24) Radiology 2007; Blodgett TM, et al. PET/CT: form and function. 242: 360-385
(25) Radiographics 2007; Kim TJ, et al. Postoperative imaging of esophageal cancer: what chest radiologists need to know. 27: 409-429
(26) J Nucl Med 2007; Guo H, et al. Diagnostic and prognostic value of 18F-FDG PET/CT for patients with suspected recurrence from squamous cell carcinoma of the esophagus. 48: 1251-1258
(27) Radiology 2007; Margolis DJA, et al. Molecular imaging techniques in body imaging. 245: 333-356
(28) Radiographics 2007; Bruzzi JF, et al. PET/CT of esophageal cancer: its role in clinical management. 27: 1635-1652
(29) AJR 2008; Yamabe Y, et al. Tumor saging of advanced esophageal cancer: combination of double-contrast esophagography and contrast-enhanced CT. 191: 753-757
(30) J Nucl Med 2009; Ben-Haim S, Ell P. 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. 50: 88-99
(31) Radiographics 2009; Kim TJ, et al. Multimodality assessment of esophageal cancer: preoperative staging and monitoring response to therapy. 29: 403-421
(32) Radiology 2010; Kwee RM. Prediction of tumor response to neoadjuvant therapy in patients with esophageal cancer with use of 18F-FDG PET: a systematic review. 254: 707-717
(33) J Nucl Med 2010; Malik V, et al. Early repeated 18F-FDG
PET
scans
during
neoadjuvant
chemoradiation
fail to predict histopathologic response or survival benefit in
adenocarcinoma of the esophagus. 51: 1863-1869
(34) J Nucl Med 2011; Meyer zun Buschenfelde C, et al. 18F-FDG
PET-guided
salvage
neoadjuvant
radiochemotherapy
of
adenocarcinoma of th esophagogastri junction: the MUNICON II
trial. 52: 1189-1196
(35) J Nucl Med 2012; Hatt M, et al. Impact of partial-volume
effect correlation on the predictive and prognostic value of
baseline 18F-FDG PETimages in esophageal cancer. 5:
12-20
(36) J Nucl Med 2012; Barber TW, et al. 18F-FDG
PET/CT Has a high impact on patient management and provides
powerful prognostic stratification in the primary staging of
esophageal cancer: A prospective study with mature survival data.
53: 864-871
(37) J Nucl Med 2012; Evaluation
of
response to neoadjuvant chemotherapy for esophageal cancer: PET
response criteria in solid tumors versus response evaluation
criteria in solid tumors. 53: 872-880
(39) Radiographics 2013; Lewis
RB, et al. Esophageal neoplasms: radiologic-pathologic
correlation. 33: 1083-1108
(40) J Nucl Med 2014; Moon SH, et al. Prediction of
occult lymph node metastasis by metabolic parameters in patients
with clinically N0 esophageal squamous cell carcinoma. 55: 743-748
(41) Radiographics 2014; Hong SJ, et al. New TNM
staging system for esophageal cancer: what chest radiologists need
to know. 34: 1722-1740
(42) J Nucl Med 2015; Goense L, et al. Diagnostic
performance of 18F-FDG PET and PET/CT for the
detection of recurrent esophageal cancer afetr treatment with
curative intent: a systematic review and meta-analysis. 56:
995-1002
(43) J Nucl Med 2016; van Rossum PSN, et al. The
incremental value of subjective and quantitative assessment of 18F-FDG
PET for the prediction of pathologic complete response to
preoperative chemoradiotherapy in esophageal cancer. 57: 691-700
(44) J Nucl Med 2017; Findlay JM, et al. Predicting
pathologic response of esophageal cancer to neoadjuvant
chemotherapy: the implications of metabolic nodal response for
personalized therapy. 58: 266-275
(45) J Nucl Med 2017; Beukinga RJ, et al.
Predicting response to neoadjuvant chemoradiotherapy in esophageal
cancer with textural features derived from pretreatment 18F-FDG
PET/CT imaging. 58: 723-729
(46) J Nucl Med 2017; Xi M, et al. 18F-FDG
PET response after induction chemotherapy can predict who will
benefit from subsequent esophagectomy after chemoradiation therapy
for esophageal adenocarcinoma. 58: 1756-1763
(47) Radiology 2018; Beukinga RJ, et al. Prediction
of response to neoadjuvant chemotherapy and radiation therapy with
baseline and restaging 18F-FDG PET imaging biomarkers
in patients with esophageal cancer. 287: 983-992
(48) AJR 2020; Banks KP, et al. It's about quality,
not quantity: qualitative FDG PET/CT criteria for therapy response
assessment in clinical practice. 215: 313-324
(49) AJR 2020; Jayaprakasam VS, et al. Role of imaging in esophageal cancer management in 2020: update for radiologists. 215: 1072-1084